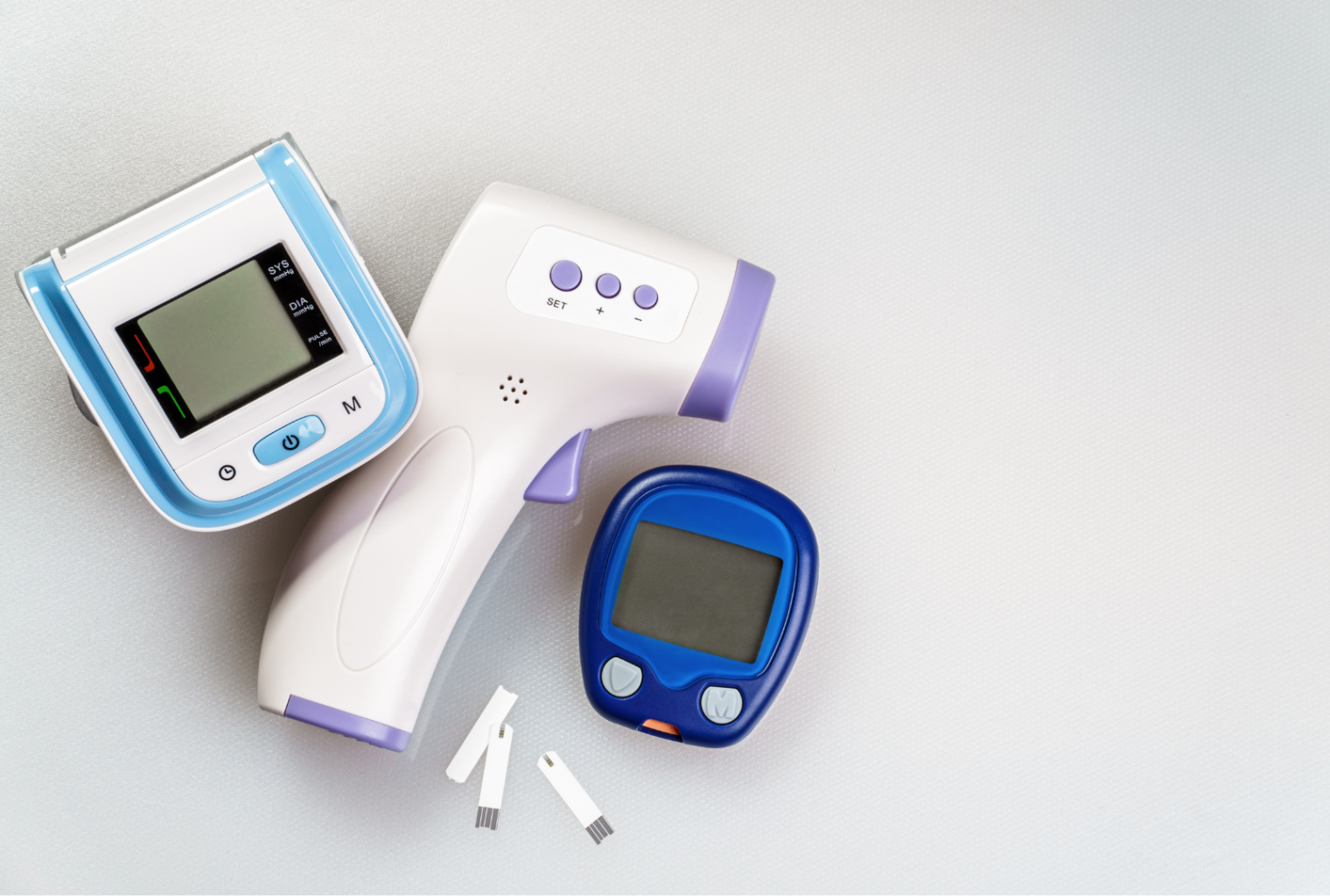
Advanced design and manufacturing of all types of medical equipment
Medical Devices
BigD is responsible for researching, designing, prototyping, and manufacturing medical devices at the forefront of medicine.
Why bigD?
We offer innovative and quality solutions to make your company stand out in the industry.
Innovation
in the outcome and the process
We strive to innovate both in the outcome and the work process to achieve the best results.
Rigour
for achieving excellence
We pay attention to every detail in design. Everything matters, and nothing can be left to chance if we want to create unique experiences.
Collaboration
the path to innovation
We understand that collaboration is essential to tackle complex projects and achieve innovative solutions.
Care
for our clients and our team
Our projects and clients deserve the utmost attention, respect, and care to create an optimal experience.
The medical devices industry grows by more than 5% annually.
- If it continues, it is expected to reach a value of $800 billion by 2030.
- There is an increasing demand for new innovative portable devices.
- Devices not designed for constant technological evolution will lose power in the market.
When designing a quality medical device, bigD focuses on 16 key factors.
1. Functionality and Purpose
The device must fulfill its intended purpose and offer technological features that bring clinical value and improve healthcare.
2. Interoperability
If the device interacts with other systems or devices, it must integrate effectively and securely share data.
3. Artificial Intelligence and Algorithms
If the device uses AI or algorithms, it must be designed so that the results and decisions generated are clear, explainable, and reliable.
4. User Experience
The design should be intuitive and easy to use for healthcare professionals, patients, and other users.
5. Cybersecurity
Devices must be protected against cyber threats and comply with information security standards to protect patient data.
6. Regulatory Compliance
Devices must comply with local and international regulations and standards, including those related to medical technology and cybersecurity.
7. Testing and Validation
Comprehensive testing and clinical validation are necessary to demonstrate the safety and effectiveness of the technological device.
8. Privacy and Consent
If the device collects patient data, it must respect privacy and informed consent in accordance with applicable regulations.
9. User Interface Design
The user interface should be clear, easy to navigate, and present information in a understandable and useful manner.
10. Updates and Maintenance
It must be possible to securely and efficiently update and maintain the device’s software and technology.
11. Scalability
The design should allow for scalability to accommodate future updates and expansions.
12. Data Analysis
If the device collects data, it must have the capacity for effective analysis to obtain valuable and useful insights.
13. Training and Education
Users must receive necessary training to understand and correctly use the technological features of the device.
14. Warranty and Support
Provide strong warranties and efficient technical support to address issues and inquiries.
15. Clinical Impact
Evaluate how the technological device will impact clinical practice, decision-making, and the quality of care.
16. Multidisciplinary Collaboration
Involve medical professionals, engineers, designers, and other experts in the design and development of the device.
By considering these key factors in the design of medical devices, safe, effective, and reliable products can be created that significantly contribute to healthcare.
RESEARCH
We analyze the initial idea or existing product and conduct research to enhance and optimize the design.
DESIGN
We brainstorm, create initial sketches with aesthetics and functionality in mind, and present the renders.
DEVELOPMENT
We bring the concept to life, focusing on parts, materials, etc., and manufacture initial prototypes to assess their viability.
ADVANCED MANUFACTURING
We have various advanced manufacturing tools that allow us to create functional prototypes.
OUR RESULTS

Before working with bigD

After working with bigD

Before working with bigD

After working with bigD

Success stories
Many talk, few act, but only the best prove. Here, we share real stories from our clients.
Some clients improving day by day with bigD





















TESTIMONIALS
“We chose bigD mainly for the clarity in their work process, their enthusiasm in taking on the project, and for the attention to detail they showed with their own brand”.
Javier ArizcurenR&D Engineer – Ingeteam

“We are very satisfied with the work done, and the experience with bigD throughout the process has been very positive”.
Joseba UlayarCEO – Ulartec

Previous
Next
Want to know more?